Medical Clinics of North America
Volume 81 � Number 3 � May 1997
Copyright � 1997 W. B. Saunders Company
Renal Disease
Anil K. Mandal MD
Potassium (K+ ) is the major intracellular cation. The total K+ content of the body averages 3500 mEq (50 mEq/kg body weight), 90% of which is intracellular. About 8% of the total body K+ is in bone and cartilages. Although only 2% of the total body potassium is in the extracellular fluid, its concentration is finely regulated by the flux of K+ between the extracellular and intracellular spaces (internal potassium homeostasis). When this concerted balance is offset, however, significant changes occur in serum potassium levels.
Daily minimum requirement of K+ is approximately 1600 to 2000 mg (40 to 50 mEq) (40 mg = 1 mEq). Potassium intake varies widely according to the type of diet consumed, age, and race. Thus, 15- to 20-year-olds may consume up to 3400 mg (85 mEq) of K+ daily, whereas elderly individuals, especially if they live alone or are disabled and are not eating balanced meals, have low K+ intake. People who eat large amounts of fruits and vegetables have a high K+ intake, on the order of 8000 to 11,000 mg (200 to 275 mEq) per day. [26] Urban whites consume about 2500 mg (62.5 mEq) of K+ per day, [17] whereas African-Americans are reported to have a low intake of K+ on the order of 1000 mg (25 mEq) per day. [12] Human milk contains small amounts of K+ , about 500 mg (12.8 mEq) per liter, whereas cow's milk contains almost three times as much, 1365 mg (35 mEq) per liter.
In a balanced state of body potassium, 80% of potassium intake is excreted by the kidneys, 15% of potassium is excreted by the gastrointestinal tract, and the remaining 5% of potassium is excreted in the sweat. Urinary potassium is for the most part secretory potassium. Distal potassium secretion is regulated by the amount of sodium in the lumina of the distal and collecting tubules, flow of urine in these segments of the nephron, and the aldosterone activity. Serum potassium in and of itself is an important factor in the regulation of aldosterone activity. The kidneys are the major regulator of external potassium homeostasis (or balance); therefore, excessive loss through the kidneys or retention because of loss of excretory function of the kidneys eventually leads to hypokalemia or hyperkalemia.
From the Section of Nephrology, Department of Veterans Affairs Medical Center; and the Department of Medicine, Wright State University, Dayton, Ohio
Address reprint requests to
Anil K. Mandal, MD
Department of Medicine (IIIW)
VA Medical Center
4100 West Third Street
Dayton, OH 45428
REGULATION OF INTERNAL POTASSIUM HOMEOSTASIS
A number of factors affect the internal potassium homeostasis; among these factors, acid-base status, plasma insulin concentration, and plasma catecholamine levels are important. Aldosterone activity has a minor role in internal potassium homeostasis.
Acid-Base Status
Extracellular acidosis or acidemia produces hyperkalemia because of efflux of potassium from inside of the cells as a result of cellular buffering of [H+ ] ions, whereas extracellular alkalosis or alkalemia produces hypokalemia because of influx of potassium into the cells. This is not an inevitable phenomenon and is exemplified by hypokalemia in proximal (type II) and classic or distal (type I) renal tubular acidosis (RTA). Hyperkalemia is a common accompaniment of type IV distal RTA. It is important to know, however, that in metabolic acidosis, potassium load is not well tolerated, unless there is a potassium leak as in proximal, or distal (type I), RTA.
In respiratory acid-base disorders, the situation is different. Both acute and chronic respiratory alkalosis have only slight effects on internal and external potassium balance and show a mild tendency to hypokalemia. Conversely, in chronic respiratory acidosis, high P CO2 and enhanced bicarbonate reabsorption are accompanied by excessive urinary potassium loss and hypokalemia.
Insulin
Insulin stimulates intracellular uptake of potassium and can rapidly reduce serum potassium concentration. This generally occurs after a high carbohydrate meal in individuals with normal islet cell function. In diabetes mellitus, in contrast, deficiency of insulin results in inhibition of intracellular uptake of potassium, which increases the propensity to hyperkalemia. This proneness to hyperkalemia, however, is generally offset by the osmotic diuresis and accompanying kaliuresis. Therefore, uncomplicated diabetics generally do not develop hyperkalemia. Hyperkalemia is common when diabetics develop hyporeninemic hypoaldosteronism or renal insufficiency.
Catecholamines
The catecholamine effect on potassium distribution is mediated by beta2 -receptors. Hence, beta2 stimulation or agonists decrease serum potassium or ameliorate a hyperkalemic response, whereas beta2 antagonists (blockers) increase serum potassium and exaggerate a hyperkalemic response to a given potassium load. The alpha-adrenergic system has an opposing effect to the beta-adrenergic system. In general, alpha-adrenergic stimulation exaggerates the hyperkalemic response, and alpha-adrenergic blockade blunts the hyperkalemic response to a given potassium load.
Beta-receptor stimulation causes hypokalemia mainly by intracellular translocation of potassium and offers a protective mechanism against potential hyperkalemia in post surgery state and renal failure. In this context, use of beta-blockers to treat hypertensives who also have advanced renal failure may not be safe. It is important to reiterate that among all the factors, renal excretion of potassium, whether high or low, determines most cases of serum potassium abnormalities.
HYPOKALEMIA
Normal serum potassium ranges from 3.5 to 5.5 mEq/L. Serum potassium less than 3.5 mEq/L is hypokalemia. Common causes of hypokalemia include:
- Diuretics.
- Alcoholism.
- Gastrointestinal disorders (vomiting, gastric suction, diarrhea).
- Renal tubular acidosis type I and II.
- Primary hyperaldosteronism.
- Secondary hyperaldosteronism (renovascular hypertension, renin-secreting tumor).
- Bartter's syndrome.
- Antibiotics, including penicillin, carbenicillin, aminoglycoside, amphotericin.
- Magnesium depletion.
- Trauma.
- Drugs other than diuretics, such as albuterol and theophylline.
- Miscellaneous causes, such as myelocytic or myelomonocytic leukemia and adrenocorticotropic hormone (ACTH)-producing bronchogenic tumor.
Causes of Hypokalemia
Diuretic therapy
Diuretic therapy is the most common cause of hypokalemia. Hypokalemia in diuretic therapy is the result of excessive loss of potassium in the urine (kaliuresis). All diuretics--thiazides, loop diuretics, and carbonic anhydrase inhibitors--produce kaliuresis and hypokalemia of variable severity. Hyponatremia is also common, in particular with indapamide. [5] Hypokalemia does not develop until total exchangeable body potassium is depleted, however, which may take several weeks. Therefore, appearance of hypokalemia shortly after initiation of diuretic therapy suggests a preexisting K+ -depleted state, such as primary or secondary hyperaldosteronism, which potentiates the hypokalemic effect of the diuretic. It should be noted that hypokalemia is infrequent in hypertensive patients treated with a diuretic, whereas hypokalemia is comparatively more common in patients with nephrotic syndrome, cirrhosis of the liver, or congestive heart failure treated with the equivalent dose of a diuretic and consuming almost the same amount of potassium in the food.
Alcoholism
Alcoholics admitted to the hospital reveal a panorama of metabolic disturbances consisting of hypokalemia, hyponatremia, hypophosphatemia, and hypomagnesemia. Hypokalemia is probably multifactorial from poor intake, gastric loss through vomiting, and renal loss resulting from activation of the renin-angiotensin-aldosterone axis.
Pyloric Stenosis and Gastrointestinal Suction and Drainage
Patients with pyloric stenosis and gastrointestinal suction and drainage following surgery frequently develop hypokalemia. Hypokalemia is partly due to gastric loss of potassium but in most part is due to renal loss of potassium from volume depletion and activation of the renin-angiotensin-aldosterone axis.
Renal Tubular Acidosis
Type I and type II RTA are commonly associated with kaliuresis and hypokalemia. In type II RTA, kaliuresis is due to increase in the delivery of bicarbonate to the distal secretory sites, resulting in increased potassium secretion. In type I RTA, in which hydrogen secretion is defective, excessive potassium secretion occurs to facilitate sodium reabsorption. In addition, loss of sodium distally causes volume depletion and activation of the renin-angiotensin-aldosterone axis, which may be offset by increased sodium intake.
Primary or Secondary Hyperaldosteronism
Hypokalemia found in a hypertensive patient who is not taking a diuretic or denies laxative abuse should alert a physician about the possibility of primary hyperaldosteronism (aldosterone-producing tumor) or secondary hyperaldosteronism resulting from renal artery stenosis or renin-secreting tumor.
Magnesium Depletion
Hypomagnesemia is a concomitant feature of hypokalemia in patients treated with a loop diuretic, in alcoholics, and in patients with malabsorption syndrome. Magnesium wasting also occurs during gentamicin and cisplatin therapy. The mechanism(s) by which hypomagnesemia causes hypokalemia is unknown; however, it should be stressed that hypomagnesemia could delay correction of hypokalemia.
Hypokalemia Associated with Ectopic Adrenocorticotropic Hormone Production
Tumors, especially small cell type bronchogenic carcinoma, may produce an ACTH, which, by stimulating both glucocorticoid hormone and aldosterone production, enhances urinary potassium loss. The diagnosis is highly suspected when a patient presents with hypokalemia, metabolic alkalosis, and hyperpigmentation. Hypokalemia can be intractable, and large amounts of potassium supplement are required to control the hypokalemia.
Hypokalemia Induced by Antibiotics
Many antibiotics, including penicillin, carbenicillin, and gentamicin, produce hypokalemia. Hypokalemia is due to excessive secretion of potassium and accompanied by kaliuresis, which occurs to dissipate luminal electronegativity caused by anionic penicillin or carbenicillin. The mechanism of hypokalemia caused by gentamicin therapy, however, is different. Renal cortical tissue after gentamicin administration demonstrates excessive numbers of lysosome. The lysosomes have been found to be the storage source of lysozyme implying the lysosome to lysozymuria and consequently hypokalemia. Amphotericin B produces hypokalemia by inducing (type I) RTA.
Bartter's Syndrome
Bartter's syndrome is chiefly found in children and young adults. Short stature, failure to thrive, muscle weakness or cramps, polyuria, and nocturia are the usual presenting features. The characteristic features include hypokalemia, hyperreninemia, hyperaldosteronism, and hyperplasia of the juxtaglomerular apparatus, but the paradox is the normal blood pressure. Normal blood pressure in this setting is considered to be due to a concomitant vasodilator hyperactivity. Subtle evidence for that is high urinary prostaglandin E2 (PGE2 ). Hypokalemia, induced in experimental animals, has been shown to produce hyperplasia of the collecting tubule cells and renal papillary interstitial cells. These cells have been shown to produce PGE2 . Therefore, it appears that hypokalemia is a primary event and that excessive PGE2 production may be a secondary event. Excessive urinary chloride (chloride > 100 mEq/L) is a characteristic feature of Bartter's syndrome. One of the sites of action of PGE2 is in the ascending thick limb of Henle's loop. Possibly PGE2 inhibits chloride reabsorption and secondary sodium (Na+ ) reabsorption and accounts for the loss of chloride and Na+ in the urine. Surreptitious diuretic use and magnesium depletion produce manifestations similar to Bartter's syndrome and are called pseudo-Bartter's syndrome.
Liddle's Syndrome
Liddle's syndrome is the reverse of Bartter's syndrome except for hypokalemia. This syndrome consists of low plasma renin activity, normal to low plasma aldosterone level, and elevation of blood pressure. The cause of this syndrome remains uncertain, although increased activity of renal Na+ , K+ -ATP-ase activity has been suggested. Supersensitivity of the mineralocorticoid because of defective tubular epithelial sodium channel has been proposed. The most recent theory in this syndrome is a defect in voltage-gated Na+ channel. [8]
Hypokalemic Familial Periodic Paralysis
This condition is characterized by intermittent episodes of sudden onset of severe muscle weakness to paralysis of the lower extremities or all four extremities. This episodic paralysis is almost invariably accompanied by severe hypokalemia, which is due to an abnormality in the internal potassium homeostasis, causing rapid shift of potassium from the extracellular space into the intracellular space. Periodic paralysis may occur as a familial condition segregating as an autosomal dominant trait. [8] The paralytic episodes may be precipitated by a high carbohydrate meal, glucose, insulin, beta-receptor agonists, or even severe anxiety associated with respiratory alkalosis and possibly outpouring of epinephrine. Drugs that circumvent against intracellular translocation of potassium, including diazoxide (inhibitor of insulin release) and beta-blockers (epinephrine antagonist), have proved to be effective therapy in familial periodic paralysis. Aldosterone antagonist can ameliorate hypokalemia and paralytic episodes.
Leukemia
Moderate to severe hypokalemia is common in acute myelocytic or myelomonocytic leukemia. Hypokalemia has been attributed to lysozymuria in these types of leukemia. The exact mechanism(s) by which lysozyme excretion induces kaliuresis and hypokalemia is unknown. Delivery of high anionic load in the distal nephron similar to penicillin or carbenicillin resulting in excessive K+ secretion has been proposed.
Chronic Abuses
Chronic ingestion of licorice as a laxative or chewing certain tobaccos can produce hypokalemia secondary to the mineralocorticoid properties of the glycyrrhizic acid they contain. Although glycyrrhizic acid itself has some mineralocorticoid activity, the primary disturbance involves inhibition of the enzyme that catalyzes the conversion of cortisol to cortisone. Therefore, cortisol level is elevated and results in kaliuresis, hypokalemia, hypervolemia, and hypertension. Plasma renin activity and plasma aldosterone levels are low, thus mimicking Liddle's syndrome. Acute renal failure following severe hypokalemic rhabdomyolysis resulting from chronic glycyrrhizin administration has been reported. It should be stressed that elderly subjects with potential volume depletion or underlying impaired renal function may be vulnerable to rapid deterioration of renal function after glycyrrhizin-induced hypokalemic rhabdomyolysis. [6]
Hypokalemia in Trauma
Hypokalemia occurs in 50% to 68% of trauma patients. Serum potassium usually decreases within 1 hour of trauma and returns to normal within 24 hours without significant potassium replacement. In an analysis, age, admission systolic blood pressure, cardiac injury, and serum epinephrine level were associated with admission serum potassium value, whereas sex, mechanism of injury, number of organ systems injured, blood glucose, serum alcohol, arterial pH, injury severity score, trauma score, estimated blood loss, and urine potassium were not significantly related to serum potassium value. In a multiple regression model, the only significant independent variables were age, arterial pH, and serum epinephrine. [34]
Immune-Related Potassium-Losing Nephropathy
Immune-related potassium-losing interstitial nephritis, immune-related distal RTA, and familial distal RTA have been associated with hypokalemia. [37] The differences are presented in Table 1 .
Pathophysiology
Significant disturbances of potassium homeostasis, hypokalemia or hyperkalemia, are often unrecognized and may cause considerable morbidity and mortality. Hypokalemia occurs because of increased Na+ , K+ -
IMMUNE-RELATED HYPOKALEMIC DISORDERS
| TABLE 1 -- IMMUNE-RELATED HYPOKALEMIC DISORDERS |
|
IRPLIN |
IRdRTA |
FdRTA |
| Sex |
Confined to women |
Confined to women |
Both sexes |
| Age |
Adults |
Adults |
Younger |
Renal potassium
wasting |
Striking feature |
Common but not a striking feature |
Common but not a striking feature |
| Hypokalemia |
Marked |
Less marked |
Less marked |
| Urinary acidification |
Normal or minimal
defect |
Marked defect |
Marked defect |
| Metabolic acidosis |
None or minimal |
Very common |
Very common |
| Nephrocalcinosis |
None |
Common |
Common |
| Renal bone disease |
None |
Common |
Common |
| IRPLIN = Immune-related potassium-losing interstitial nephritis; IRdRTA = immune-related distal renal tubular acidosis; FdRTA = familial distal renal tubular acidosis. |
ATP-ase activity (e.g., beta2 -agonist, theophylline, or insulin poisoning), competitive blockade of K+ channels (e.g., barium or chloroquine poisoning), gastrointestinal and renal losses, or alkalosis. [
4]
Potassium being the major intracellular cation, muscles and renal tubule cells are most severely affected. Skeletal muscles are more involved than heart muscles. Generalized muscle weakness, paralytic ileus, electrocardiogram (ECG) changes (flat or inverted T waves, prominent U waves, ST segment depression), and cardiac arrhythmias (atrial tachycardia with or without block, atrioventricular dissociation, ventricular tachycardia, ventricular fibrillation) may occur. Two patients are presented here to illustrate the adverse effects of hypokalemia on the heart and skeletal muscles.
Heart Muscles
WH, a 68-year-old white man, was referred to the renal clinic for hypokalemia in preparation of his eye surgery. He is regularly followed in the hypertension clinic for isolated systolic hypertension but received no antihypertensive drug treatment. He is an alcoholic. On June 3, 1996, a serum chemistry during routine follow-up in hypertension clinic showed glucose 180 mg/dL, blood urea nitrogen (BUN) 12 mg/dL, creatinine 1 mg/dL, sodium 138 mEq/L, potassium 3.4 mEq/L, chloride 98 mEq/L, and CO2 (representing bicarbonate) 25.8 mEq/L. An ECG showed ST-T changes, prolonged Q-T interval, and T-U fusion. These ECG changes were considered to be due to hypokalemia (Fig. 1) . He was receiving a potassium chloride supplement 20 mEq orally twice daily, which was increased to 20 mEq three times daily. Two weeks later, a repeat serum chemistry showed glucose 101 mg/dL, BUN 14 mg/dL, creatinine 0.9 mg/dL, sodium 139 mEq/L, potassium 3.8 mEq/L, chloride 98 mEq/L, and CO2 26.4 mEq/L. Thus, in 2 weeks, his serum potassium increased by 0.4 mEq/L, which was accompanied by
 Figure 1. An electrocardiogram shows ST-T flattening in Lead 1, AVL, V4 , V5 , and V6 and prolonged QT interval or T-U fusion. His serum potassium was 3.4 mEq/L.
Figure 1. An electrocardiogram shows ST-T flattening in Lead 1, AVL, V4 , V5 , and V6 and prolonged QT interval or T-U fusion. His serum potassium was 3.4 mEq/L.
a decrease of 79 mg/dL serum glucose and reversal of ECG changes to normal patterns
(Fig. 2) .
Hypokalemia is a common problem in the elderly and is caused by low dietary intake of potassium (tea and toast) and drugs. Among the drugs, the most common one is a diuretic. In patients with congestive heart failure receiving digitalis or with acute myocardial infarction, hypokalemia is an established risk factor and may lead to cardiac arrhythmias and even sudden death. [16] It should be noted that a sudden or rapid decrease of serum potassium below 2.5 mEq/L carries the risk of dangerous arrhythmias and requires immediate replacement therapy. [29]
Skeletal Muscles
Myopathy may develop with hypokalemia, resulting in weakness or paralysis of the extremities. Worsening of the symptoms with exercise tends to support the diagnosis of hypokalemic myopathy. The lower extremities are affected more than the upper extremities. In untreated severe hypokalemia (serum potassium < 2.5 mEq/L), myopathy may progress onto rhabdomyolysis and myoglobinuria, and acute renal failure may supervene. These myopathic complications are more common in hypokalemia caused by alcoholism than in hypokalemia resulting from other causes.
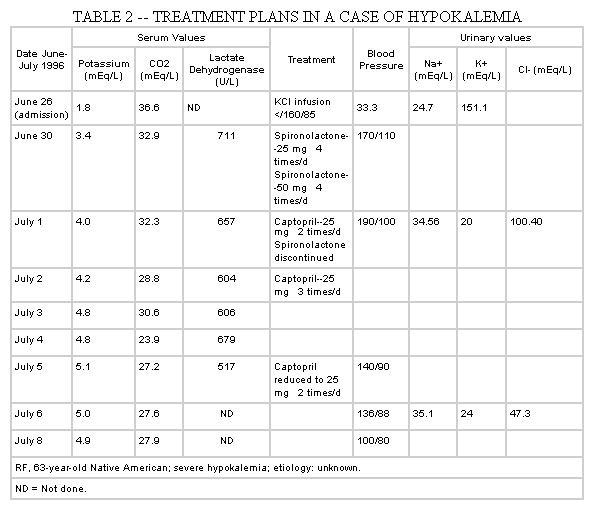
RF, a 63-year-old Native American, was transferred to Dayton VA Medical Center from a community hospital on June 26, 1996, with the complaints of pain and weakness of both legs and falling down for 3 days. There was no other significant history in the past except history of hypertension for which he was not taking any medication. The only significant medicine he was taking that may relate to his problem was albuterol (a beta-agonist), two puffs every 6 hours. Admission laboratory studies showed serum glucose 103 mg/dL, serum sodium 149 mEq/L, serum potassium 1.8 mEq/L, serum chloride 101 mEq/L, serum CO2 36.6 mEq/L, BUN 10 mg/dL, and serum creatinine 0.9 mg/dL. His hemoglobin and hematocrit were 14 g/dL and 42%. Spot urinary electrolytes were sodium 33.3 mEq/L, potassium 24.7 mEq/L, and chloride 151.1 mEq/L. Urine osmolality was 639 mOsm/kg. Muscle enzymes, including aspartate transaminase (AST), lactate dehydrogenase, and creatine phosphokinase (CPK), were all elevated. CPK was most markedly elevated; initial CPK level was 6711 U/L, which increased to 11,351 U/L the following day. MB fraction was within normal limit. ECG showed nonspecific ST-T wave changes. His urine myoglobin was positive. He had severe hypokalemia, which resulted in myopathy and rhabdomyolysis. Three months before this admission, his serum potassium was normal (4.4 mEq/L).
The cause of his hypokalemia remained undetermined. Beta-agonist albuterol may have caused hypokalemia in this patient. High urinary chloride suggested Bartter's syndrome. Plasma renin and aldosterone levels were drawn. Initially, he was treated with intravenous infusion
 Figure 2. After treatment with potassium chloride supplement 20 mEq three times daily for two weeks, his serum potassium increased to 3.8 mEq/L. A repeat electrocardiogram showed a normal pattern.
Figure 2. After treatment with potassium chloride supplement 20 mEq three times daily for two weeks, his serum potassium increased to 3.8 mEq/L. A repeat electrocardiogram showed a normal pattern.
of potassium, 60 mEq potassium chloride (KCl) in 1 L of fluid, then switched to oral KCl supplements. After 4 days (June 30, 1996), he felt stronger in the legs, his serum potassium increased to 3.4 mEq/L, and sodium and CO2 decreased to 140 and 32.9 mEq/L. Bartter's syndrome was suspected, and spironolactone was added to the regimen, but it did not help and was discontinued. His blood pressure increased rapidly to the level of 190/100 to 110 mm Hg, when captopril was added in the dosage of 25 mg twice daily on July 1, 1996. Captopril increased to 25 mg three times daily on July 2, 1996. On July 3, 1996, his serum potassium was 4.5; on July 5, 1996, serum potassium was 5.1. Captopril was reduced to 25 mg twice daily. His serial potassium levels and treatment profiles are presented in
Table 2 .
His plasma renin activity was 0.25 ng/mL/hour and plasma aldosterone level less than 2.5 mug/dL. These values refute a diagnosis of Bartter's syndrome and primary or secondary hyperaldosteronism. These values, however, suggest the possibility of Liddle's syndrome or surreptitious use of licorice.
There are many individual reports on glycyrrhizin (licorice)-induced hypokalemic myopathy (GIHM). A cumulative report comprised 32 men, 25 women, and 2 patients without record of sex; the average age was 55.2 years. In many cases, conditions leading to the onset of GIHM were habitual licorice ingestion; ingestion of antituberculous agents containing licorice; and long-term ingestion of licorice-containing agents for chronic gastritis, chronic hepatitis, or chronic dermatitis. The combined use of diuretic agents increased the risk of GIHM in an overwhelming number of cases. The main presenting feature was flaccid quadriplegia in almost all patients. Laboratory findings included a mean potassium level of 1.98 mEq/L, a mean CPK of 5385.7 IU/L, a mean aldosterone concentration of 2.92 mug/dL (n = 2 to 13 mug/dL), and a mean plasma renin activity of 0.17 ng/mL/hour (n = 0.8 to 4.4 ng/mL/hour). Complete cure was attained in 57 of 59 cases of GIHM by stopping ingestion of glycyrrhizin (licorice) and giving a potassium supplement. [30]
In cases with obscure hypokalemia, the following possibilities should be considered: (1) diuretic abuse, (2) surreptitious vomiting, and (3) laxative abuse. The distinguishing features between diuretic abuse, surreptitious vomiting, and laxative abuse are presented in Table 3 .
Effect of Potassium Depletion on Renal Function
Potassium depletion causes variable renal function impairment. In chronic hypokalemia, both urine diluting and urine concentrating ability are significantly impaired. [1] In potassium-depleted subjects, renal function has been evaluated by creatinine clearance technique during both induced hypotonic polyuria and subsequent moderate antidiuresis induced by low-dose infusion of lysine-8-vasopressin (LVP). In hypokalemic subjects, reduction in creatinine clearance (in the absence of significant reduction in blood pressure), inhibition of the fractional reabsorption of chloride by diluting segments, and depression of the diuretic
response to water load were noted. Further in potassium-depleted subjects, LVP was less effective in reducing creatinine clearance, whereas it was effective in reducing the fractional excretion of NaCl. [
2]
Diagnosis of the Cause of Hypokalemia
In all cases of hypokalemia, a complete history of drug intake is mandatory. History of diuretic or laxative abuse, diarrhea, vomiting, or alcoholism must be ruled out. In case of hypokalemia associated with hyperchloremic metabolic acidosis, morning urine pH should be checked, and if it is consistently 6 or above, diagnosis of type I RTA becomes tenable. Further investigations include obtaining a flat film of the abdomen or ultrasound of the kidneys to look for nephrocalcinosis or nephrolithiasis; 24 hour urinary calcium for hypercalciuria; and finally, in some cases, an ammonium chloride test to confirm the diagnosis of type I RTA. In the hypertensive patient, basal plasma renin activity and plasma aldosterone level should be obtained to determine if hypokalemia is due to primary or secondary hyperaldosteronism. In primary hyperaldosteronism, basal plasma renin activity is usually below normal range and may even be in the low range, whereas plasma aldosterone level is normal or high. In secondary hyperaldosteronism, both plasma renin activity and plasma aldosterone level are normal to high. In suspected Bartter's syndrome, plasma renin activity, plasma aldosterone level, urinary chloride, and urinary PGE2 levels should be obtained. If no cause for hypokalemia is found, obscure causes such as Bartter's syndrome, Liddle's syndrome, surreptitious diuretic use, and licorice abuse must be considered. See Table 3 for the differential features.
Management
Principles of Management
If the serum K+ level is between 4 and 3.5 mEq/L, it signals potassium depletion; however, replacement of K+ is not essential at this stage. Instead, the following steps should be taken to minimize further hypokalemia: (1) The patient should be encouraged to consume potassium-rich foods such as fresh fruit, vegetables, fruit juice, and meats. (2) If the patient is receiving a diuretic, dosage of the diuretic should be reduced and, if possible, the diuretic discontinued. (3) If this low serum [K+ ] level is not diuretic induced by history, a spot urine should be analyzed for potassium. If urinary potassium is low (<20 mEq/L), hypokalemia is of extrarenal origin. Careful interrogation should be made about licorice abuse, and the possibility of malabsorption syndrome should be ruled out. If history is negative, it is wise to proceed with investigations, as stated earlier, to delineate the cause of low serum [K+ ].
The issue is unsettled whether to treat a patient whose serum K+ level is between 3.5 and 3 mEq/L and who is asymptomatic. Potassium supplementation is recommended in certain classes of patients who are vulnerable to cardiac arrhythmias. These classes include patients with congestive heart failure and receiving digitalis and those with history of myocardial infarction or ischemic heart disease.
If the serum [K+ ] level is below 3 mEq/L, potassium supplementation is essential. The following guidelines should be observed to obtain optimum results and to avoid unwarranted events.
Oral Potassium Replacement.
Oral replacement is adequate in most patients. KCl is the preparation of choice. KCl dissociates readily into [K+ ] and [Cl- ] ions allowing reabsorption of [K+ ] and Cl- ions at the expense of Na+ and HCO3 - . With the loss of HCO3 - in the urine, metabolic alkalosis is abated. This preparation is useful in all patients except in those with metabolic acidosis, in which potassium bicarbonate or potassium citrate is preferable. The average dose of KCl is 20 to 40 mEq two to four times daily depending on the severity of the depletion.
KCl is available in liquid (elixir), powder, or tablet form. The elixir is acrid in taste and is disliked by most patients; however, it is the cheapest preparation. The acrid taste of the elixir can be minimized by mixing the solution in chilled water or orange juice. A powder form called K-Lor (Abbott, Abbott Park, IL) is available in an envelope containing 20 mEq KCl. When mixed in chilled water, it has a lemonade flavor and is more acceptable than the elixir. The KCl slow-release tablet available as Kaon-CL (Adria, Kalamazoo, MI) or Slow-K (Ciba, Summit, NJ) contains 8 mEq of [K+ ]. The dosage varies from two to four tablets daily. The enteric-coated, or slow-release, tablet may produce intestinal ulceration and stenosis and may be unsafe. Potassium bicarbonate available as K-Lyte (Mead Johnson, Princeton, NJ) provides 25 mEq of [K+ ] per tablet. The usual dose is one tablet two or three times daily. In an open, randomized study, the effect of oral KCl and of potassium citrate/bicarbonate was compared in patients with hypokalemia. In both groups, 80 mEq of [K+ ] were administered daily. Serum K+ increased from 3.2 to 4 mEq/L in 4 days in both groups. Thus, increase of serum [K+ ] was not different between the two groups. Oral K+ therapy should be monitored by daily serum [K+ ] measurement. Generally, serum [K+ ] begins to rise in 72 hours or earlier. If serum [K+ ] level does not appreciably rise by 96 hours, concomitant magnesium depletion should be suspected. During potassium replacement therapy, depleted cells must be replenished with potassium before a significant rise in serum [K+ ] occurs. The renal tubular cells have Na+ , K+ pump, which intrudes [K+ ] and extrudes sodium [Na+ ]. This pump is activated by the enzyme Na+ , K+ -ATP-ase. The cofactor for this enzyme is magnesium. In a severe magnesium-deficient state, the Na+ , K+ pump is not activated, and therefore, the cells are not replenished with [K+ ]. The evidence for this is excessive loss of [K+ ] in the urine. Therefore, it is imperative to measure the serum magnesium level if serum [K+ ] level does not rise in a reasonable period. If the serum magnesium level is lower than 1 mEq/L (N = 1.7 to 2.8 mEq/L), magnesium sulfate 50% 2 mL (1 g) intramuscularly twice first day, 2 mL twice second day, and 2 mL once third day should be administered. Magnesium sulfate also is available as a 10% solution. The equivalent dose is 10 mL (1 g) instead of 2 mL of 50% solution. Alternatively, magnesium sulfate may be administered orally 3 g every 6 hours for four doses.
Potassium and magnesium deficiencies, particularly those induced by loop or thiazide diuretic therapy, have been linked in clinical studies to an increased frequency of serious arrhythmias and mortality in acute myocardial infarction. Magnesium repletion has been shown not only to increase magnesium levels, but also to increase muscle potassium and to decrease the frequency of ventricular ectopic beats. Potassium replenishment alone may have a detrimental effect in magnesium-depleted patients. The potassium-sparing diuretic spironolactone has been shown to spare both potassium and magnesium and may therefore be a more appropriate diuretic therapy in patients at cardiovascular risk. [7]
Intravenous Potassium.
Intravenous administration of potassium is recommended in hypokalemia associated with cardiac arrhythmias and rapid ventricular response, familial periodic paralysis, and severe myopathy. In these situations, a patient should be admitted in the intensive care unit, KCl 100 mEq is mixed in 1 L of normal saline, and the solution is infused at a rate of 100 mL/hour to deliver 10 mEq KCl per hour. Administration of potassium must be continuously monitored. Rarely, potassium may be infused at a rate of 20 mEq or even 40 mEq in 100 mL fluid per hour (by mixing 200 to 400 mEq KCl in a 1-L bag of saline). As soon as the cardiac rhythm returns to normal or respiratory muscle strength has been restored to normal, intravenous infusion is gradually tapered and then discontinued. Oral KCl is then initiated. Serum [K+ ] levels should be obtained during the infusion and immediately after cessation of the infusion.
In critical care situations, infusion of potassium in normal saline at a rapid rate of 20 to 40 mEq delivered over 1 hour was safe and effectively increased serum potassium levels in a dose-dependent and predictable fashion. Furthermore, these results were independent of the patient's underlying renal function or associated diuretic administration. [13] [20] [32] Intravenous infusion of potassium is fraught with great danger. In this regard, the effects of 495 sets of KCl infusions administered to a medical intensive care unit population were examined. The infusion sets consisted of one to eight consecutive individual infusions, each containing 20 mEq of KCl in 100 mL of saline administered. The mean preinfusion potassium level was 3.2 mEq/L, and the mean postinfusion potassium level was 3.9 mEq/L. The mean increment in serum potassium level per 20 mEq infusion was 0.25 mEq/L. No temporally related life-threatening arrhythmias were noted; however, there were 10 instances of mild hyperkalemia. The authors endorsed the relative safety of using concentrated (200 mEq/L) KCl infusions at a rate of 20 mEq/hour via central or peripheral vein to correct hypokalemia in patients in the intensive care unit. [19]
Caution: No matter how low the serum [K+ ] level and how alarming the cardiac arrhythmia, [K+ ] solution must never be injected directly into a vein. Human subjects, having no ability to adapt rapidly to potassium load, develop life-threatening hyperkalemia and cardiac arrest resulting in death.
Potassium-Sparing Drugs.
These drugs include spironolactone, triamterene, and amiloride. All these drugs act in the cortical and medullary collecting tubules and inhibit secretion of potassium. Spironolactone acts by inhibiting binding of aldosterone to its receptors, whereas triamterene and amiloride are effective independent of aldosterone. The mechanism(s) of action of triamterene and amiloride in retaining potassium is not clear; however, they inhibit a number of ATP-ase enzymes. The way by which inhibition of these enzymes causes retention of [K+ ] is quite intriguing. Any of these potassium-sparing agents alone may be used in congestive heart failure or nephrotic syndrome, in which high dosage of a diuretic or a combination of diuretics is required and [K+ ] loss in the urine can be high. A potassium-sparing drug is frequently used to potentiate the natriuretic and diuretic effects of a loop or thiazide diuretic and at the same time prevent its hypokalemic effect. To use a potassium-sparing agent, one must ensure that renal function is normal. Potassium-sparing agents avidly retain potassium, which in the presence of renal insufficiency frequently leads to hyperkalemia. In a small percentage of patients, life-threatening hyperkalemia may occur. Combination of [K+ ] supplement and a potassium-sparing agent is unnecessary except in congestive heart failure, nephrotic syndrome, cirrhosis of the liver, or hypokalemia caused by ectopic ACTH, in which urinary [K+ ] loss can be excessive.
Spironolactone (Aldactone; Searle, Chicago, IL) is available as 25-mg, 50-mg, or 100-mg tablets. The usual dose is 25 to 50 mg every 6 hours. The effect is not observed until after 48 hours. The maximum total dose is 400 mg/day. Common side effects include hyperkalemia, especially if [K+ ] supplement is used concomitantly and in the presence of renal insufficiency. Gynecomastia is a common adverse effect, and the severity of gynecomastia is related to the dose and duration of therapy. It is usually reversible following discontinuation of Aldactone. Somewhat less common side effects include painful breasts and decreased libido in women, impotence in men, and sodium depletion syndrome.
Triamterene (Dyrenium; SmithKline Beecham, Philadelphia, PA) is available as 50-mg or 100-mg capsules. Starting dose is 50 mg twice daily, but it may be increased up to 100 mg/twice daily. Triamterene is available as a combination product of 25 mg hydrochlorothiazide and 50 mg triamterene (Dyazide; SmithKline Beecham). This product is often used in the treatment of mild hypertension. Common side effects include hyperkalemia; sodium depletion, especially with severe salt restriction; and metabolic acidosis. Uncommon side effects consist of megaloblastic anemia, especially in cirrhotics, and renal calculi. Acute interstitial nephritis has been reported.
Amiloride (Midamor; Merck, Sharp and Dohme, West Point, PA) is available as a 5-mg tablet. The usual dosage is 5 mg daily; however, in severe hypokalemia, 10 mg daily is recommended. A combination product called Moduretic (Merck, Sharp and Dohme) contains 50 mg hydrochlorothiazide and 5 mg amiloride. The usual dose is one to two tablets in edematous conditions or in mild hypertension. Side effects are minimal; however, hyperkalemia and metabolic acidosis may occur.
Osmotic Demyelination Syndrome in Hypokalemia
Osmotic demyelination syndrome is a well-known neurologic complication following rapid correction of hyponatremia. There are several reports in which patients were hypokalemic as well as hyponatremic, and in no instance was serum potassium normalized before the time of most rapid correction of serum sodium. There is a general consensus that hypokalemia may predispose patients to develop osmotic demyelination syndrome following correction of hyponatremia. In neurologically stable patients with severe hyponatremia, it may be beneficial to correct hypokalemia before correction of serum sodium. This manipulation may further reduce the incidence of osmotic demyelination syndrome. [24]
Other Management Strategies for Hypokalemia
Beta-adrenergic blocking drugs, including propranolol, atenolol, and an investigational cardioselective beta-blocker with intrinsic sympathomimetic activity (cetamolol) have been used to treat hypokalemia. In one study, hypokalemia was induced by epinephrine infusion in normal subjects. Epinephrine-induced hypokalemia occurred in the placebo group (maximum decrease of 1 mEq/L) and atenolol group (maximum decrease of 0.59 mEq/L). ECG changes also appeared. Serum potassium rose slightly from 3.94 to 4.07 mEq/L in propranolol and cetamolol groups. ECG changes were rare. Thus, propranolol and cetamolol can prevent epinephrine-induced hypokalemia and ECG changes. [18]
Bartter's Syndrome
Bartter's syndrome can be treated by one of the following agents, alone or in combination: (1) [K+ ] supplement; (2) spironolactone; (3) beta-receptor blocker, which inhibits renin production and thereby controls hyperreninemia and hyperaldosteronism; (4) prostaglandin synthesis inhibitor; (5) angiotensin converting enzyme (ACE) inhibitor drugs, which have the same effects as beta-blockers. [K+ ] supplement and spironolactone may be administered in the dosage already stated for hypokalemia. Beta-blockers, such as propranolol, can be given orally in a dosage of 40 to 80 mg daily. An ACE inhibitor, such as captopril, appears to be the most effective therapy in Bartter's syndrome. It can be given orally in a daily dose of 25 mg two or three times daily.
Liddle's Syndrome
Liddle's syndrome can be treated by one of the following agents, alone or in combination: (1) K+ supplement, (2) amiloride or triamterene. The preparations and dose of [K+ ] supplement, amiloride, and triamterene are similar as in other hypokalemic conditions. Spironolactone is not effective in this disorder and should not be used.
Some Helpful Tips
Finally, some helpful tips are provided to facilitate treatment of hypokalemia. In edematous conditions, such as nephrotic syndrome, a perplexing situation sometimes develops in the management of edema. Not infrequently, after an initial good response to a diuretic, diuresis decreases and does not increase with increasing dose of the diuretic, even when renal function is normal. It has been mentioned earlier that edematous patients promptly develop hypokalemia with diuretic therapy, suggesting a preexisting potassium depletion state. Increasing the dosage of diuretic or substituting one diuretic for another does not help much. This diuretic-resistant state is apparently due to intracellular Na+ and fluid retention, which can be obviated by increasing the dose of [K+ ] supplement or by adding a potassium-sparing agent to the regimen. Potassium causes an efflux of intracellular Na+ , which leads to natriuresis and consequently diuresis.
HYPERKALEMIA
Hyperkalemia is not as common as hypokalemia. If all patients with acute and chronic renal failure are excluded, the incidence of hyperkalemia is rather insignificant. At the outset, it should be stressed that hyperkalemia can be pseudohyperkalemia and is caused most commonly by extravascular hemolysis. Pseudohyperkalemia also can be caused by severe leukocytosis or thrombocytosis. If severe hyperkalemia is observed as an isolated finding in an otherwise normal laboratory report of a patient, this hyperkalemia is most likely pseudohyperkalemia and is caused by hemolysis. True elevation of serum potassium is usually accompanied by other abnormal laboratory findings, singly or in combination, such as low CO2 content and most commonly elevated BUN and serum creatinine. If there was no evidence of hemolysis in the blood sample and white blood cell and platelet counts are normal, whole blood (plasma) should be analyzed for potassium. Plasma potassium is normal in the case of pseudohyperkalemia.
By definition, serum potassium greater than 5.5 mEq/L is called hyperkalemia. Although mild hyperkalemia (5.5 to 6 mEq/L) is of slight concern, moderate and severe hyperkalemia (between 6.1 and 6.9 mEq/L and  7 mEq/L) may lead to grave consequences, especially if it develops acutely. As stated earlier in the introductory section, 80% of the daily potassium intake is excreted by the kidneys. Therefore, inability of the kidneys to secrete and excrete [K+ ] as in acute and chronic renal failure could result in retention of potassium and hyperkalemia. Interestingly, kidneys retain the ability to secrete and excrete potassium despite moderate to severe renal failure. This is especially true in patients with good urine output. Thus, hyperkalemia does not always develop in patients with uncomplicated chronic renal failure. It should be stressed, however, that the delicate renal handling of potassium in chronic renal failure may be tipped off easily by slight potassium load caused by indiscriminate intake of potassium in foods, release of cellular potassium because of infection or trauma, or inhibition of tubular secretion of K+ by drugs. Imbalance in internal potassium homeostasis as in diabetes or beta-blocker therapy may result in hyperkalemia even in the presence of mild renal insufficiency. Hyperkalemia may develop under any one of four major categories of conditions:
7 mEq/L) may lead to grave consequences, especially if it develops acutely. As stated earlier in the introductory section, 80% of the daily potassium intake is excreted by the kidneys. Therefore, inability of the kidneys to secrete and excrete [K+ ] as in acute and chronic renal failure could result in retention of potassium and hyperkalemia. Interestingly, kidneys retain the ability to secrete and excrete potassium despite moderate to severe renal failure. This is especially true in patients with good urine output. Thus, hyperkalemia does not always develop in patients with uncomplicated chronic renal failure. It should be stressed, however, that the delicate renal handling of potassium in chronic renal failure may be tipped off easily by slight potassium load caused by indiscriminate intake of potassium in foods, release of cellular potassium because of infection or trauma, or inhibition of tubular secretion of K+ by drugs. Imbalance in internal potassium homeostasis as in diabetes or beta-blocker therapy may result in hyperkalemia even in the presence of mild renal insufficiency. Hyperkalemia may develop under any one of four major categories of conditions:
- Decrease in [K+ ] excretion. Potassium excretion is reduced when filtration of potassium is decreased as a result of decrease in glomerular filtration rate, secretion of potassium is inhibited as a result of deficiency of aldosterone, and secretion of potassium is inhibited by drugs.
Potassium excretion is decreased in acute and chronic renal failure. Severe hyperkalemia occurs more commonly in acute renal failure (ARF) than in chronic renal failure and is especially common in hypercatabolic ARF and rhabdomyolysis or tumor lysis syndrome accompanied by ARF. It is not common to find severe hyperkalemia in stable chronic renal failure, which may be, in part, due to enhanced excretion of potassium by the gastrointestinal tract.
Secretion of potassium is inhibited in diffuse adrenocortical insufficiency or Addison's disease, selective hypoaldosteronism, and potassium inhibited drugs. In Addison's disease, hyperkalemia is almost always accompanied by hyponatremia, hypovolemia, hypotension with appreciable decrease in blood pressure on upright posture, and renal insufficiency. Addison's disease should be suspected in all cases of hyperkalemia, particularly when accompanied by hypovolemia and mild renal insufficiency. Aldosterone deficiency does not occur in adrenal insufficiency secondary to pituitary failure.
Hyperkalemia is the hallmark of selective hypoaldosteronism, and a serious cardiac arrhythmia or cardiac arrest resulting from hyperkalemia often draws attention to the problem. Hypoaldosteronism may be idiopathic or secondary to hyporeninemia because it is commonly observed in diabetics or in patients with chronic tubulointerstitial nephritis. In patients normokalemic at baseline, hyperkalemia developed with a frequency of 0.59% in 20,809 nondiabetics but 1.08% in 922 diabetics. Hyperkalemia is 3 to 5 fold higher in those more than 60 years of age and diabetics. [14]
Four discrete groups of drugs that preferentially inhibit [K+ ] secretion are potassium-sparing drugs, prostaglandin synthesis inhibitors, ACE inhibitors, and miscellaneous drugs. Hyperkalemia occurs with increased frequency in elderly diabetics when a potassium-sparing combination diuretic is used. [14] Miscellaneous drugs include trimethoprim-sulfamethoxazole (Bactrim), ketorolac tromethamine, heparin, and pentamidine. There are several reports on the association of trimethoprim alone or trimethoprim-sulfamethoxazole and hyperkalemia. [11] [35] In studies involving humans and rats, trimethoprim increased serum K+ concentration by 0.6 mEq/L despite normal adrenocortical function and glomerular filtration rate. Serum K+ levels greater than 5 mEq/L were observed in 50% of patients studied. [35] In rats, trimethoprim infusion inhibited renal K+ excretion by 40% and increased renal Na+ excretion by 46%. This study has elegantly shown that trimethoprim acts similar to amiloride and blocks apical membrane sodium channels in the mammalian distal nephron. [35] It should be noted that high-dose trimethoprim-sulfamethoxazole therapy used for the treatment of Pneumocystis carinii pneumonia in human immunodeficiency virus-infected patients leads to an increase in serum K+ levels and may result in life-threatening hyperkalemia. Patients receiving trimethoprim-sulfamethoxazole require close monitoring of their serum K+ levels, particularly 7 to 10 days after the start of therapy. [11] Ketorolac tromethamine (Toradol), an injectable nonsteroidal anti-inflammatory drug commonly used for relief of postoperative pain, can give rise to ARF, hyperkalemia, or both. These complications are reversible. [28]
Prolonged use of heparin can cause aldosterone suppression, natriuresis, and decreased excretion of potassium. Greater than normal serum K+ levels occur in about 7% of patients, but marked hyperkalemia generally requires the presence of additional factors perturbing K+ balance, such as diabetes, renal insufficiency, and use of other K+ retaining drugs. Therefore, serum K+ should be monitored in patients being given heparin for 3 or more days and particularly in patients with high risk for hyperkalemia as stated earlier. [27] The cause of heparin-induced hyperkalemia is rather obscure. Hypoaldosteronism has been suggested but not documented. [10] In an experimental study, the author has found that heparin given chronically by subcutaneous injection in rats caused hyperreninemia in both normotensive and spontaneously hypertensive rats. Serum aldosterone level, however, was elevated only in normotensive rats but significantly reduced in spontaneously hypertensive rats. [33] Although nonselective beta-blocking agents including propranolol have been known to produce hyperkalemia, labetalol, a combination of alpha-blocking and beta-blocking drug, has been reported to cause hyperkalemia as well. [3] Drugs causing hyperkalemia are listed in Table 4 .
- Increase in potassium intake (load). Excretion of potassium load may be greatly delayed when renal function is reduced. Thus, in patients with chronic renal failure (nondialyzed) and in dialyzed patients, indiscriminate consumption of potassium-rich foods is a frequent cause of severe or life-threatening hyperkalemia. Sudden death among dialyzed patients who were noncompliant in diet had been attributed to hyperkalemia. In tumor lysis syndrome and rhabdomyolysis, massive release of potassium occurs from the intracellular space; however, hyperkalemia does not occur unless ARF supervenes. Similarly, trauma, intravascular hemolysis, transfusion of stored blood, and catabolic state such as infection or high fever are associated with release of potassium from the cells; however, hyperkalemia is uncommon as long as renal function is normal and normal to high urine output is maintained with fluid therapy.
- Tubular unresponsiveness to aldosterone. In certain conditions, such as sickle cell anemia, systemic lupus erythematosus, amyloidosis, and renal transplantation, hyperkalemia is considered to be due to a defect in the tubular secretion of potassium. This defect has been ascribed to tubular unresponsiveness to aldosterone, possibly as a result of a defect in the receptor binding of aldosterone. Plasma renin activity and plasma aldosterone levels are usually normal. Tubules respond to exogenous mineralocorticoids by retention of sodium and water. The mechanism of this aldosterone unresponsiveness relative to potassium secretion is unknown.
- Redistribution of potassium. Efflux of potassium from the intracellular space to the extracellular space can occur in a variety of conditions. This includes metabolic acidosis as in renal failure, diabetic ketoacidosis, lactic acidosis, digitalis overdosage, use of heparin, infusion of arginine or lysine chloride for the treatment of metabolic alkalosis, or infusion of hypertonic solution such as 50% dextrose or mannitol. Hypertonic solution increases the osmolality of the extracellular space, which causes dryness or even necrosis of the cells and leak of potassium from the intracellular space to the extracellular space. Similar to hypokalemic periodic paralysis, there is an entity called hyperkalemic periodic paralysis. This is due to efflux of potassium from the intracellular space to the extracellular space. Finally, beta-receptor blocking drugs can cause hyperkalemia by inhibiting the effect of epinephrine.
TABLE 4 -- DRUG-RELATED HYPERKALEMIA
| Family of Agents |
Individual Drugs |
Potassium-sparing agents alone or in
combination with a diuretic |
Spironolactone (most)
Triamterene
Amiloride (least)
Triamtrene-hydrochlorothiazide (least) |
| Angiotensin converting enzyme inhibitors |
Captopril
Enalapril
Fosinopril (least) |
| Nonsteroidal anti-inflammatory agents |
Indomethacin (least)
Ibuprofen (most)
Ketorolac |
| Anti-infective |
Trimethoprim-sulfamethoxazole (Bactrim)
(common)
Pentamidine |
| Anticoagulant |
Heparin (uncommon) |
| Cardiac glycoside |
Digitalis (uncommon) |
| Antihypertensives |
Beta-blockers (uncommon)
Alpha- and beta-blockers (labetalol)
(uncommon) |
Pseudohyperkalemia may be more common than true hyperkalemia. Life-threatening hyperkalemia occurs, most commonly in chronic renal failure settings especially in patients who indiscriminately consume potassium-rich foods or use salt substitute or in those patients who receive a potassium-sparing agent, beta-blocking drugs, or ACE inhibitors. Hyporeninemic hypoaldosteronism is recognized increasingly as a common cause of hyperkalemia among elderly diabetics or patients with advanced vascular disease or some type of chronic tubulointerstitial disease (type IV RTA). Addison's disease should be suspected in all patients with obscure hyperkalemia.
Diagnosis
Clinical manifestations of hyperkalemia are fewer but more severe than those of hypokalemia. Occasionally a patient is brought to the emergency department with a history of chest pain resembling acute myocardial infarction or because the patient had cardiac arrest. Generalized muscular weakness and paresthesias in the hands and feet are not uncommon complaints. Hyperkalemic periodic paralysis may occur. Usually there is a history of diabetes, hypertension, or chronic renal disease.
The ECG is most rewarding. The changes consist of tall peaked T wave (>5 mm) with serum [K+ ] level, 6 to 7 mEq/L; widening of QRS complex and smaller amplitude of P wave with serum [K+ ] level, 7 to 8 mEq/L; and fusion of QRS complex with T wave forming sine waves with serum [K+ ] level, 8 to 9 mEq/L. When serum K+ level increases more than 9 mEq/L, atrioventricular dissociation, ventricular tachycardia, or ventricular fibrillation and death may ensue.
A total of 220 consecutive patients admitted to the university-affiliated Urban County Hospital with a diagnosis of renal failure or hyperkalemia were evaluated for ECG changes of hyperkalemia. Eighty-seven patients had hyperkalemia, and 133 did not. When patients with moderate to severe hyperkalemia only were analyzed, sensitivities of the readers were 0.62 and 0.55. The readers' ability to predict the severity of hyperkalemia was equally poor. The authors concluded that ECG is not a sensitive method of detecting hyperkalemia. The specificity of the ECG is better for hyperkalemia, but empiric treatment of hyperkalemia based on the ECG alone leads to mistreatment of at least 15% of patients. [36]
Serum chemistry shows in almost all patients with hyperkalemia variable degrees of azotemia and metabolic acidosis. The latter tends to aggravate hyperkalemia.
In all patients with hyperkalemia, especially in those with recurrent or chronic hyperkalemia, investigations must be done to determine the cause of hyperkalemia. The following are stressed: A history of intake of salt substitute (contains KCl and NH4 Cl), potassium supplement, potassium-sparing agent, beta-blocking drug, ACE inhibitor drug, or prostaglandin synthesis inhibitor must be obtained. In patients with mild hyperkalemia, especially those with hyponatremia and postural hypotension, a morning (6 A. M.) serum cortisol level should be obtained. If this serum cortisol level is low (N = 7 to 25 mug/dL) (serum cortisol level is highest in early morning specimen), a cortisone stimulation test should be done. A cortisone stimulation test can be done by infusion of 50 U of ACTH in 1 L of saline continuously for 8 hours and measurement of the serum cortisol level after completion of infusion. Alternatively a synthetic ACTH analogue cosyntropin (Cortrosyn) 0.25 mg may be injected intravenously and serum cortisol level obtained before and 30 minutes after Cortrosyn. In either test, a normal individual shows increase in plasma cortisol level by at least 10 mug/dL from the baseline level, whereas in Addison's disease, no or slight elevation in plasma cortisol level is observed. In patients with a long history of diabetes mellitus or history suggestive of some type of tubulointerstitial renal disease, plasma renin activity and plasma aldosterone level may be obtained.
Management
Mild to Moderate Hyperkalemia (Serum [K+ ]
5.5 to <7 mEq/L)
Recommended therapy includes the following: A low-potassium diet (50 to 60 mEq/day) can be instituted. Salt substitute, potassium supplement, potassium-sparing agent, heparin, beta-blocking agent, ACE inhibitor, or prostaglandin synthesis inhibitor drug can be discontinued. If a patient is hypertensive, hypertension can be treated with a sympathetic inhibitor, alpha-blocker (prazosin), or calcium channel blocker. Sodium polystyrene sulfonate (Kayexalate; Winthrop-Breon, New York, NY) can be prescribed. This agent is a cation exchange resin; in vivo, 1 g of the resin exchanges 1 mEq Na+ for 1 mEq K+ in the intestines. It can be given orally or rectally (enema). Oral route is more effective than enema. As the resin passes through the intestines, cations exchange with those cations that are in greater concentration, and the cationically modified resin is excreted in the feces. Because of the relatively high concentration of potassium present in the large intestine, conversion of the resin to the potassium form occurs principally at this site.
Kayexalate is available in powder form or as a suspension in 70% sorbitol, which provides 5 g Kayexalate in 20 mL suspension. The recommended oral dose is 20 to 40 mL four times daily or until serum potassium level is less than 5.5 mEq/L. Then it should be given as a maintenance dose of 20 mL two or three times daily. Kayexalate given in powder form can produce anorexia, nausea, vomiting, and constipation. The last-mentioned is most troublesome, especially in elderly patients. Most of these symptoms, however, are alleviated by suspending Kayexalate in 70% sorbitol.
If oral administration is not tolerated, Kayexalate may be administered rectally as a retention enema. Kayexalate powder 60 to 100 g can be administered suspended in 100 to 200 mL 30% sorbitol or 10% dextrose and warmed up to body temperature. Alternatively, 120 to 180 mL of the commercially available suspension may be administered as a retention enema, after the suspension has been warmed up to body temperature. After an initial cleansing enema, a soft large (28 F) rubber tube should be inserted about 20 cm into the rectum and taped in place. The Kayexalate suspension is then administered rectally by gravity. The tube may then be flushed with 50 to 100 mL of normal saline or half-normal saline, clamped and left in place. If back leakage occurs, the patient's back should be elevated. The suspension may be retained for 60 minutes or for several hours, after which the rectum is washed with clear water.
For metabolic acidosis, which is generally an accompaniment of hyperkalemia, administration of sodium bicarbonate or sodium citrate (is converted into bicarbonate in the body) is desirable. Sodium bicarbonate 600 mg tablet two or three times daily or a mixture of sodium citrate and citric acid (Shohl's solution) should be prescribed. Shohl's solution is more acceptable than sodium bicarbonate. One milliliter of Shohl's solution contains 1 mEq Na+ , and citrate after metabolic degradation produces 1 mEq HCO3 . The usual dose is 10 to 15 mL three times daily. Care must be taken in not causing Na+ overloading, which leads to hypervolemia, hypertension, and possibly congestive heart failure. The problem of Na+ retention, however, may be minimized by the concomitant use of a loop diuretic, provided that the patient has some residual renal function. Excessive use of Shohl's solution may lead to metabolic alkalosis and hypokalemia. Additionally, sodium citrate is an anticoagulant, which might increase the propensity to bleeding in uremia and excessive absorption of aluminum if aluminum hydroxide gel is concomitantly used as a phosphate binder.
Severe Hyperkalemia (Serum [K+ ] >7 mEq/L)
Wide QRS complex, sine wave, or ventricular tachycardia in the ECG is considered an emergency. It should be mentioned, however, that serum potassium level alone is not the sole determining factor for the cardiotoxicity of hyperkalemia; the rapidity of the rise of serum K+ also contributes to cardiotoxic effect. Management in this emergency is discrete and consists of the following measures in rapid succession, in the following order:
Calcium gluconate infusion is available as 10% solution in 10-mL ampule. Twenty milliliters must be pushed intravenously directly in 5 to 10 minutes, and the same doses may be repeated after 1 to 2 minutes, if necessary. Calcium antagonizes the membrane excitability induced by hyperkalemia and abolishes the ventricular arrhythmias. It should be noted, however, that calcium does not lower serum K+ level. The action of calcium is immediate, and it lasts for a few minutes. Therefore, intravenous push should be followed by a calcium infusion, in which 5 ampules are added into a 500-mL bottle of 10% dextrose or 5% dextrose and normal saline solution and infused slowly.
Traditionally, sodium bicarbonate infusion was a popular therapy to control severe hyperkalemia. The idea was that bicarbonate increases the pH and by translocating K+ intracellularly lowers serum K+ level. Although many cases of hyperkalemia have associated metabolic acidosis, this is not the rule, as in dialysis patients, in whom severe hyperkalemia may develop without concomitant severe metabolic acidosis. In these cases, bicarbonate infusion is not an effective therapy. In patients with end-stage renal disease (ESRD) without or with dialysis treatment, severe hyperkalemia can be most effectively treated by (1) glucose and insulin and (2) salbutamol. Several reports are consistent in that regard. [21] [22] [23] [25] It was observed that hyperkalemia occurs during fasting in ESRD probably as a result of insulinopenia and suggests that diminished response to epinephrine may contribute to hyperkalemia. [9] A study compared the effectiveness of salbutamol 0.5 mg intravenously in 15 minutes (group A); glucose 40 g intravenously plus 10 units insulin intravenously in 15 minutes (group B); and salbutamol 0.5 mg intravenously, glucose 40 g intravenously, and insulin 10 U intravenously over a 15-minute period (group C). Serum K+ was measured at 30, 60, 180, and 360 minutes later. All treatments reduced serum K+ , maximal at 30 or 60 minutes and ranging from -0.5 � -0.1 to -1.5 � -0.2 mEq/L. Patients in group C exhibited a significantly greater decrement in serum K+ when compared to group B. There were significant differences between groups A and C. [21] Salbutamol is effective by intravenous infusion (0.5 mg) or nebulization (10 mg) according to most studies. [21] [22] Some authors, however, suggest that intravenous salbutamol is preferred in ESRD requiring a rapid lowering of serum K+ , whereas nebulization is preferred in ESRD patients with associated coronary artery disease because heart rate is less elevated with nebulization than with intravenous therapy. [23]
Dialysis Therapy
Because most severe hyperkalemia is usually encountered in patients with ARF or ESRD, dialysis treatment is essential in these patients and should be instituted promptly. In the event a facility for dialysis therapy does not exist or the patient has to be transported a great distance for dialysis, treatment with Kayexalate in sorbitol must be initiated immediately. In case of severe hyperkalemia, the recommended initial oral dose is 60 mL, then 40 mL every 6 hours until serum K+ is less than 6 mEq/L, then 20 mL three times daily. An excessive amount of sorbitol causes diarrhea, which further enhances K+ excretion.
Hypoaldosteronism
Hyperkalemia tends to be chronic in selective hypoaldosteronism or hyporeninemic hypoaldosteronism. This chronic hyperkalemia in hypoaldosteronism may be treated by Kayexalate, alkali, furosemide, and most effectively by 9a-fluorohydrocortisone acetate (Florinef Acetate, Squibb, Princeton, NJ), a synthetic analogue of aldosterone. It is available as a 0.1-mg tablet. The usual dose is 0.05 mg to 0.3 mg daily. It is safe to begin with the smallest dose and gradually increase to a maximum dose of 0.3 mg daily monitored by regular examination for blood pressure and body weight. The drug is administered once daily. It has been shown that a mineralocorticoid such as Florinef lowers serum K+ by extrarenal mechanisms and can be safely used in ESRD patients. [31]
Addison's Disease
If Addison's disease is confirmed, treatment should be initiated with hydrocortisone 37.5 mg daily. The total dose may be divided into 30 mg in the morning and 7.5 mg in the evening.
References
1. Agnoli GC, Borgatti R, Cacciari M, et al: Interactions between the renin-angiotensin system and prostanoids in modulating renal function in potassium-depleted healthy women. Prostaglandins Leukot Essent Fatty Acids 50:347, 1994
2. Agnoli GC, Borgatti R, Cacciari M, et al: Studies on renal function in healthy women with different degrees of induced potassium depletion patterns of hypokalemic renal dysfunction. Boll Soc Ital Biol Sper 69:557, 1993
3. Arthus S, Greenberg A: Hyperkalemia associated with intravenous labetalol therapy for acute hypertension in renal transplant recipients. Clin Nephrol 33:269, 1990
4. Bradberry SM, Vale JA: Disturbance of potassium homeostasis in poisoning. J Toxicol Clin Toxicol 33:295-310, 1995
5. Chan TY: Indapamide-induced severe hyponatremia and hypokalemia. Ann Pharmacother 29:1124-1128, 1995
6. Chubachi A, Wakui H, Asakura K, et al: Acute renal failure following hypokalemic rhabdomyolysis due to chronic glycyrrhizic acid administration. Intern Med 31:708-711, 1992
7. Dyckner T: Relation of cardiovascular disease to potassium and magnesium. Am J Cardiol 65:K44, 1990
8. George AL: Hereditary dysfunction of voltage-gated sodium channels: From clinical phenotypes to molecular mechanisms. Nephrol Dial Transplant 11:1730, 1996
9. Gifford JD, Rutsky EA, Kirk KA, et al: Control of serum potassium during fasting in patients with end stage renal disease. Kidney Int 35:90, 1989
10. Gonzalez-Marlin G, Diaz-Molinas MS, Martinez AM, et al: Heparin-induced hyperkalemia: A prospective study. Int J Clin Pharmacol Ther Toxicol 29:446, 1991
11. Greenberg S, Reiser IW, Chous Y, et al: Trimethoprim-sulfamethoxazole induces reversible hyperkalemia. Ann Intern Med 119:291, 1993
12. Grimc, Luft FC, Miller JZ, et al: Racial differences in blood pressure in Evans County, Georgia: Relationship to sodium and potassium intake and plasma renin activity. J Chronic Dis 33:87, 1980
13. Hamil RJ, Robinson LM, Wexler HR, et al: Efficacy and safety of potassium infusion therapy in hypokalemic critically ill patients. Crit Care Med 19:694, 1991
14. Hollenberg NK, Mickiewicz C: Hyperkalemia in diabetes mellitus: Effect of a triamterene-hydrochlorothiazide combination. Arch Intern Med 149:1327, 1989
15. Howes LG: Which drugs affect potassium? Drug Saf 12:240-244, 1995
16. Isaac G, Holland OB: Drug-induced hypokalemia. Drugs Aging 2:35, 1992
17. Khaw KT, Barrett-Connor E: Dietary potassium and stroke-associated mortality: A 12-year prospective population study. N Engl J Med 316:235, 1987
18. Klausner MA, Irwin C, Mullane JF, et al: Effect of cetamolol on epinephrine-induced hypokalemia. J Clin Pharmacol 28:751, 1988
19. Kruse JA, Carlson RW: Rapid correction of hypokalemia using concentrated intravenous potassium chloride infusions. Arch Intern Med 150:613, 1990
20. Kruse JA, Clark VL, Carlson RW, et al: Concentrated potassium chloride infusions in critically ill patients with hypokalemia. J Clin Pharmacol 34:1077, 1994
21. Lens XM, Montoliu J, Cases A, et al: Treatment of hyperkalemia in renal failure: Salbutamol v. insulin. Nephrol Dial Transplant 4:228, 1989
22. Liou HH, Chiang SS, Wu SC, et al: Hypokalemic effects of intravenous infusion or nebulization of salbutamol in patients with chronic renal failure: Comparative study. Am J Kidney Dis 23:266, 1996
23. Liou HH, Chiang SS, Wu SC, et al: Intravenous infusion or nebulization of salbutamol for treatment of hyperkalemia in patients with chronic renal failure. Chung Hua I Hsueh Tsa Chih 53:276, 1994
24. Lohr JW: Osmotic demyelination syndrome following correction of hyponatremia: Association with hypokalemia. Am J Med 96:408, 1994
25. McClure RJ, Prasad VK, Brocklebank JT: Treatment of hyperkalemia using intravenous and nebulized salbutamol. Arch Dis Child 70:126, 1994
26. National Research Council: Diet and Health: Implications for Reducing Chronic Disease Risk: Report of the Committee on Diet and Health, Food and Nutrition Board. Washington, DC, National Academy Press, 1989
27. Oster JR, Singer I, Fishman LM: Heparin-induced aldosterone suppression and hyperkalemia. Am J Med 98:575, 1995
28. Pearce CJ, Gonzalez FM, Wallin JD: Renal failure and hyperkalemia associated with ketorolac tromethamine. Arch Intern Med 153:1000, 1993
29. Schaefer M, Link J, Hannenmann L, et al: Excessive hypokalemia and hyperkalemia following head injury. Intens Care Med 21:235, 1995
30. Shintani S, Murase H, Tsukagoshi H, et al: Glycyrrhizin (licorice)-induced hypokalemic myopathy. Eur Neurol 32:44, 1992
31. Singhal PC, Desroches L, Mattana J, et al: Mineralocorticoid therapy lowers serum potassium in patients with end stage renal disease. Am J Nephrol 13:138, 1993
32. Singhis S, Gautham S, Lal A: Safety and efficacy of a concentrated potassium chloride solution infusion for rapid correction of hypokalemia. Indian Pediatr 31:565, 1994
33. Susic D, Mandal AK, Jovovic DJ, et al: Antihypertensive action of heparin: Role of the renin-angiotensin-aldosterone system and prostaglandins. J Clin Pharmacol 33:342, 1993
34. Vanek VW, Seballos RM, Chong D, et al: Serum potassium concentrations in trauma patients. South Med J 87:41-46, 1994
35. Velazquez H, Perazella MA, Wright FS, et al: Renal mechanism of trimethoprim-induced hyperkalemia. Ann Intern Med 119:296, 1993
36. Wrenn KD, Slovis CM, Slovis BS: The ability of physicians to predict hyperkalemia from the ECG. Ann Emerg Med 20:1229, 1991
37. Wrong OM, Feest TG, MacIver AG: Immune-related potassium-losing interstitial nephritis: A comparison with distal renal tubular acidosis. QJM 86:513-534, 1993



